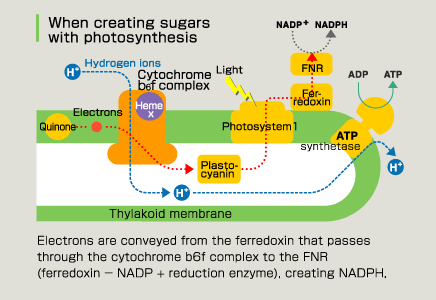
It is said that a structural analysis of the photosynthesis protein cytochrome b6f complex is difficult with the huge membrane protein, but it has been isolated from blue-green algae and its three-dimensional structure identified. Surprisingly, when this was done, a third heme was found in addition to the two b-hemes that were predicted from spectrographic research. This is not in the respiratory cytochrome bc1 complex, whose three-dimensional structure already has been identified. This was named “heme x” and was studied.
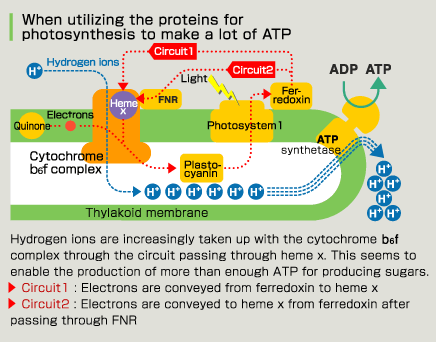
Photosynthetic reaction proceeds even without heme x. This seems to be because it is related to the route that gives preference to ATP acquisition, not sugar synthesis, despite being in the chloroplast. Energy—specifically ATP—is required to overcome the difficult living environment of plants and blue-green algae, which has a high salt concentration. Plants are different than animals because they cannot flee when their environment deteriorates. That is probably why they use heme x for the preferential creation of ATP. It is very typical of organisms that they add different procedures to the proteins (cytochrome) related to energy production, the basis for living, to respond to differences in the environment and ways of life, rather than create something new. This unexpected discovery required the observation of proteins with x-ray crystal structure analysis. |
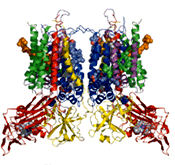 |
The three-dimensional structure of the cytochrome b6f complex
The unanticipated heme x was found on the edge of the portion buried in the membrane. |
|