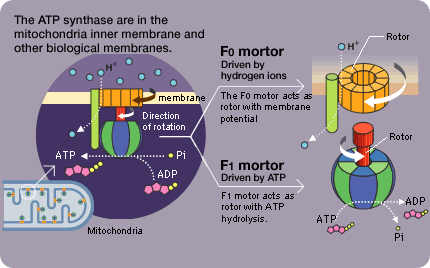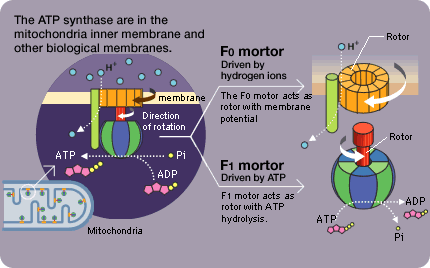
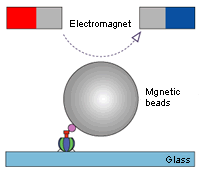
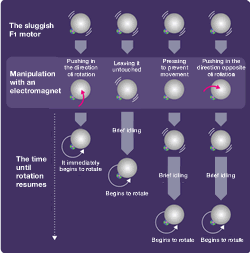
We made an unexpected discovery when observing the movement of the F1 molecule. For example, during the F1's rotation, the rotation is sometimes suspended and the molecule briefly idles (rotational Brownian motion). Rotation resumes about 30 seconds later. Unless the ADP resulting from the decomposition of the ATP is separated, the next ATP decomposition reaction will not begin and rotation will stop. Pressing the F1 that seems to be lagging in the direction of rotation will cause rotation to resume immediately. If it is pressed in the opposite direction, however, it will briefly remain motionless. The ADP affinity weakens in the direction of rotation and strengthens in the opposite direction (the direction of ATP synthesis).
This is a superb mechanism in which the ATP breakup and synthesis are both accomplished with a single motor. Stopping the lagging F1 at the idling center of rotation prevents rotation from resuming. If it happens to be jolted in the direction of rotation while idling, the ADP would be separated and rotation would likely resume.
The molecule is addressed by operating a single molecule, and its behavior is carefully observed. The reaction is different for each molecule. This tests the abilities of the scientist, who must determine how to organize this for science, which demands repeatability. This may well create a narration for science in the future. |